
Photos courtesy of Burnette.īurnette’s story is an unusual one: After a quick dip into acting school after high school, he went through the academic training mill. Army after serving 35 years in various positions. Neal Burnette retired in 2005 as a colonel in the U.S. Because Burnette didn’t bestow his name on the blot, it’s likely that the current generation of investigators don’t know he was involved in developing the technique that is now ubiquitous in molecular biology and biochemistry research laboratories and used as a clinical diagnostic for HIV-AIDS. Eventually the journal agreed to publish the paper, which now has been cited more than 6,000 times. The paper initially was rejected by the journal Analytical Biochemistry, but it went viral among molecular biologists as a preprint.

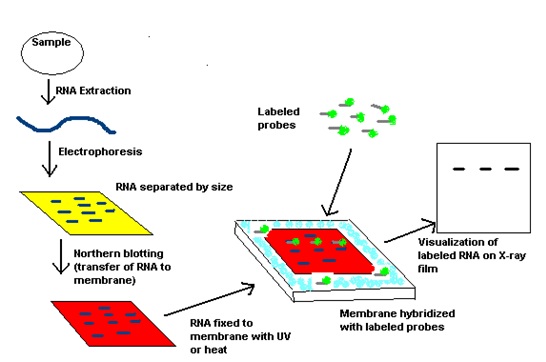
Neal Burnette published a paper that described a technique called Western blotting (1). (1975): "Detection of specific sequences among DNA fragments separated by gel electrophoresis", J Mol Biol., 98:503-517.Thirty-one years ago, W. It also allows for the fixation of the target-probe hybrids, required for analysis by autoradiography or other detection methods. The transfer step of the DNA from the electrophoresis gel to a membrane permits easy binding of the labeled hybridization probe to the size-fractionated DNA. Hybridization of the probe to a specific DNA fragment on the filter membrane indicates that this fragment contains DNA sequence that is complementary to the probe.
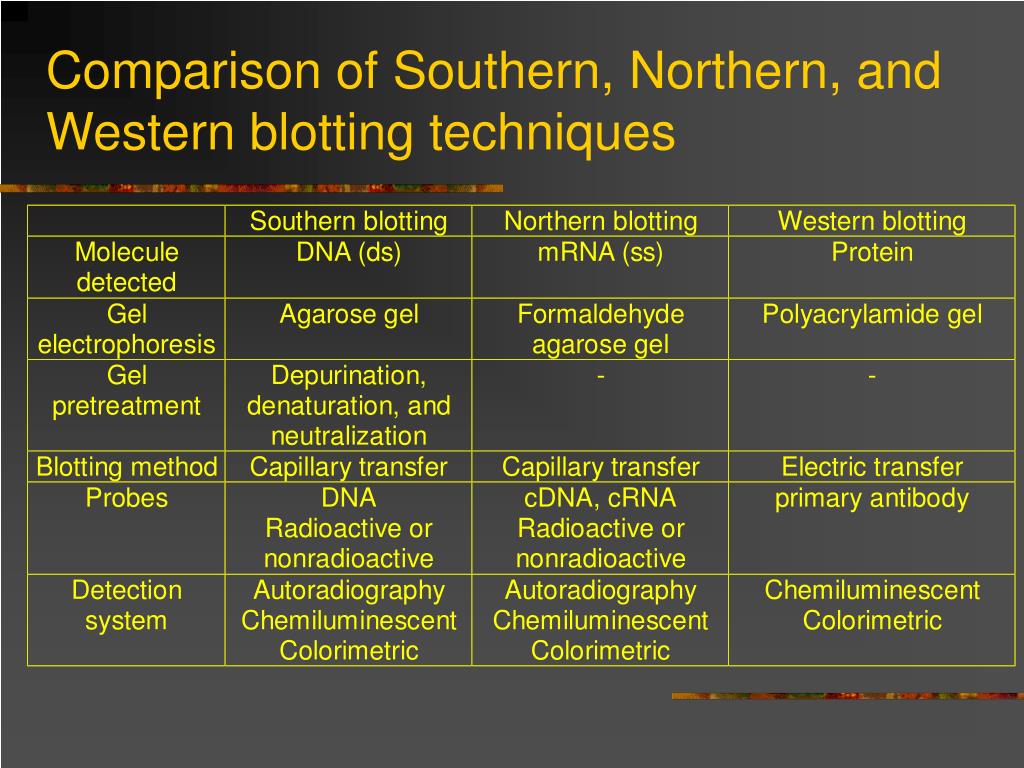
A sheet of nitrocellulose (or, alternatively, nylon) membrane is placed on top of (or below, depending on the direction of the transfer) the gel.The denaturation in an alkaline environment provides for improved binding of the negatively charged DNA to a positively charged membrane, separates it into single DNA strands for later hybridization to the probe (see below), and destroys any residual RNA that may still be present in the DNA. If alkaline transfer methods are used, the DNA gel is placed into an alkaline solution (typically containing sodium hydroxide) to denature the double-stranded DNA.If some of the DNA fragments are larger than 15 kb, then prior to blotting, the gel may be treated with an acid, such as dilute HCl, which depurinates the DNA fragments, breaking the DNA into smaller pieces, thus allowing more efficient transfer from the gel to membrane.The DNA fragments are then electrophoresed on an agarose gel to separate them by size.Restriction endonucleases are used to cut high-molecular-weight DNA strands into smaller fragments.


 0 kommentar(er)
0 kommentar(er)
